First Class Material
Ceramic has been used as implant material for decades. Due to their excellent biocompatibility, chemical stability and high wear resistance, alumina-based ceramic has successfully helped to restore function and relieve pain.
Ceramic components from CeramTec owe their superior properties to a unique material composition, the most modern production techniques and a multi-stage, comprehensive system of quality control.
Currently, we offer components in two ceramic materials: BIOLOX®delta, the 4th and latest generation of bioceramics for joint replacement and BIOLOX®forte monolithic alumina.

BIOLOX®delta
BIOLOX®delta has become a true benchmark for ceramic material in arthroplasty. It is an alumina matrix composite ceramic with high fracture strength1, excellent wear properties2 and outstanding biocompatibility.3
The introduction of BIOLOX®delta in 2003 opened new horizons, making complex geometries and a wider range of future orthopedic innovations possible: revision heads, knee implants, spinal implants, and even hip resurfacings. Since then, the BIOLOX® pink ceramic has been on the rise. BIOLOX®delta has more than 20 years of successful clinical experience.
Explore the story behind BIOLOX®delta
Download the BIOLOX®delta Quick Reference Guide
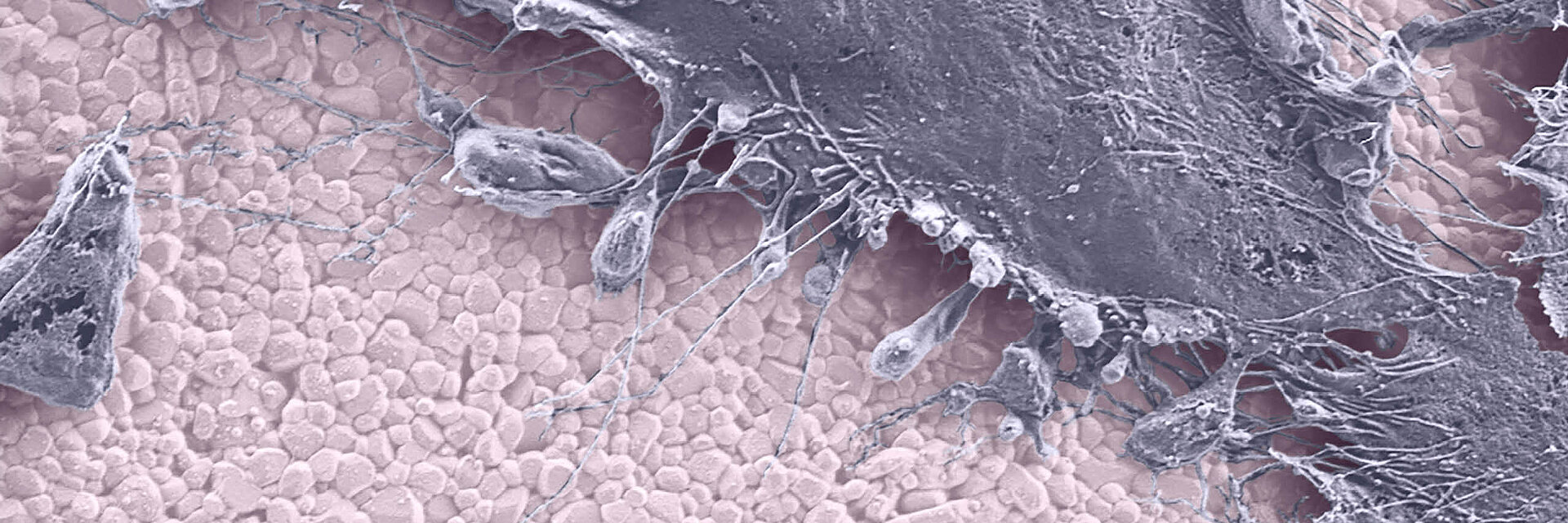
Fewer Immune Reactions
Today's analytical methods provide more accurate analyses of biological interactions between wear products and the immune system. Only recently has technology allowed the precise characterization of wear debris and observation of immune cell behavior in the presence of clinically relevant wear particles.
The biocompatibility of alumina matrix composites like BIOLOX®delta has been proven, clinically, in in-vitro studies and in in-vivo animal studies.3,9,13,18 The BIOLOX®delta particles fail to stimulate an inflammatory response and do not cause any DNA damage or oxidative stress in human cells in clinically relevant doses. In other words, the material and its debris are not cytotoxic and/or genotoxic.3
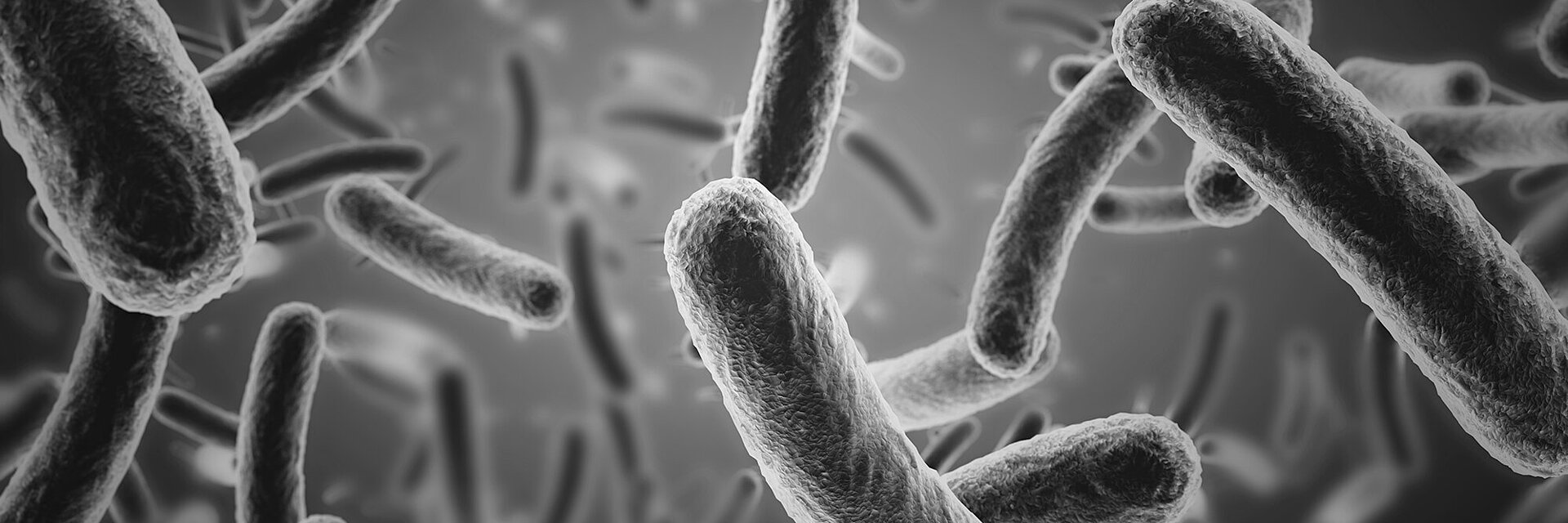
Fewer Bacterial Infections
Recent in-vitro studies and analyses of explants have demonstrated that the bacterial adhesion is reduced on BIOLOX® ceramic surfaces. Additionally, studies showed a reduced biofilm formation on BIOLOX® surfaces compared to metal and polymer surfaces.5,6
These findings resonate with current observations in the clinical field, suggesting that ceramic bearings reduce the risk of revision for periprosthetic infection.7,8
1. Kurtz SM, Kocagöz S, Arnholt C, Huet R, Ueno M, Walter WL. Advances in zirconia toughened alumina biomaterials for total joint replacement. J Mech Behav Biomed Mater. 2014;31:107-116. doi:10.1016/j.jmbbm.2013.03.022.
2. Grupp TM, Holderied M, Mulliez MA, et al. Biotribology of a vitamin E-stabilized polyethylene for hip arthroplasty - Influence of artificial ageing and third-body particles on wear. Acta Biomater. 2014;10(7):3068-3078. doi:10.1016/j.actbio.2014.02.052.
3. Asif I M. Characterisation and Biological Impact of Wear Particles from Composite Ceramic Hip Replacements. [PhD thesis]. Leeds, UK: University of Leeds; 2018. etheses.whiterose.ac.uk/20563. Accessed March 6, 2020.
4. Hopf F, Thomas P, Sesselmann S, et al. CD3+ lymphocytosis in the peri-implant membrane of 222 loosened joint endoprostheses depends on the tribological pairing. Acta Orthop. 2017;88(6):642-648. doi:10.1080/17453674.2017.1362774.
5. Trampuz A, Maiolo EM, Winkler T, Perka C. Biofilm formation on ceramic, metal and polyethylene bearing components from hip joint replacement systems. Orthopaedic Proceedings 2016;98-B (SUPP 10):80.
6. Sorrentino R, Cochis A, Azzimonti B, et al. Reduced bacterial adhesion on ceramics used for arthroplasty applications. J Eur Ceram Soc. 2018;38(3):963-970. doi:10.1016/j.jeurceramsoc.2017.10.008.
7. Pitto RP, Sedel L. Periprosthetic joint infection in hip arthroplasty: Is there an association between infection and bearing surface type? Clin Orthop Relat Res. 2016;474(10):2213-2218. doi:10.1007/s11999-016-4916-y.
8. Lenguerrand E, Whitehouse MR, Beswick AD, et al. Risk factors associated with revision for prosthetic joint infection after hip replacement: a prospective observational cohort study. Lancet Infect Dis. 2018;18(9):1004-1014. doi:10.1016/S1473-3099(18)30755-2.
9. Maccauro G, Cittadini A, Magnani G, et al. In vivo characterization of Zirconia Toughened Alumina material: a comparative animal study. Int J Immunopathol Pharmacol. 2010;23(3):841-846. doi:10.1177/039463201002300319.
10. Cunningham BW, Hallab NJ, Hu N, McAfee PC. Epidural application of spinal instrumentation particulate wear debris: a comprehensive evaluation of neurotoxicity using an in vivo animal mode. J Neurosurg Spine. 2013;19:336-350. doi:10.3171/2013.5.SPINE13166.
11. Piconi C, Porporati AA, Streicher RM. Ceramics in THR bearings: Behavior under off-normal conditions. Key Eng Mat. 2014;631:3-7. doi:10.4028/www.scientific.net/kem.631.3.
12. Zietz C, Bergschmidt P, Lange R, Mittelmeier W, Bader R. Third-body abrasive wear of tibial polyethylene inserts combined with metallic and ceramic femoral components in a knee simulator study. Int J Artif Organs. 2013;36(1):47-55. doi:10.5301/ijao.5000189
13. Kretzer JP, Mueller U, Streit MR, et al. Ion release in ceramic bearings for total hip replacement: Results from an in vitro and an in vivo study. Int Orthop. 2018;42(1):65-70. doi:10.1007/s00264-017-3568-1.
14. Tsaousi A, Jones E, Case CP. The in vitro genotoxicity of orthopaedic ceramic (Al2O3) and metal (CoCr alloy) particles. Mutat Res. 2010;697(1-2):1-9. doi:10.1016/j.mrgentox.2010.01.012.
15. Esposito C, Maclean F, Campbell P, Walter WL, Walter WK, Bonar SF. Periprosthetic tissues from third generation alumina-on-alumina total hip arthroplasties. J Arthroplasty. 2013;28(5):860-866. doi:10.1016/j.arth.2012.10.021.
16. Higuchi Y, Hasegawa Y, Seki T, Komatsu D, Ishiguro N. Significantly lower wear of ceramic-on-ceramic bearings than metal-on-highly cross-linked polyethylene bearings: A 10- to 14-year follow-Up study. J Arthroplasty. 2016;31(6):1246-1250. doi:10.1016/j.arth.2015.12.014.
17. Higuchi Y, Seki T, Morita D, Komatsu D, Takegami Y, Ishiguro N. Comparison of wear rate between ceramic-on-ceramic, metal-on-highly cross-linked polyethylene, and metal-on-metal bearings. Rev Bras Ortop (Sao Paulo). 2019;54(3):295-302. doi:10.1055/s-0039-1691762.
18. Porporati AA, Piconi C, Mettang M, Deisinger U, Reinhardt C, Pitto R. 12 - Ceramics for artificial joints: The relevance of material biocompatibility. In: Osaka A, Narayan R, eds. Bioceramics From Macro to Nanoscale. Elsevier Series on Advanced Ceramic Materials. Elsevier; 2021. doi:10.1016/B978-0-08-102999-2.00012-0. Accessed November 10, 2020.
We support you