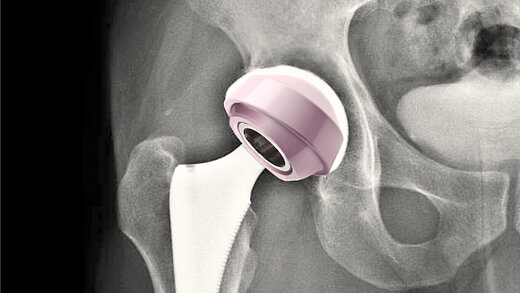
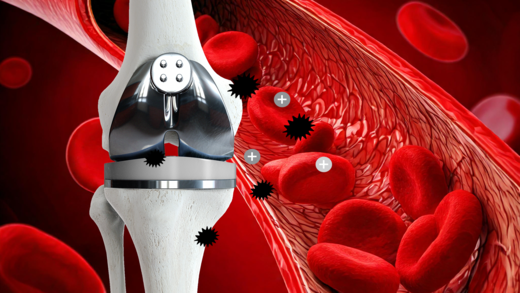
In today’s healthcare environment, the demand for advanced, biocompatible joint replacement solutions for patients is high, while the pressure on healthcare systems is increasing. The need for innovative, cost-effective medical products is undeniably urgent.
CeramTec is committed to providing superior, yet cost-effective solutions tailored to the needs of today’s patients and healthcare challenges.
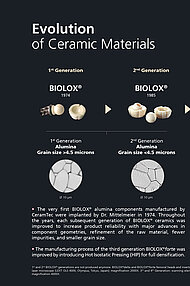
Since 1974, we have supplied close to 30 million BIOLOX® components worldwide, establishing a track record of outstanding product performance and the best clinical outcomes backed by robust evidence.
But our ambitions do not stop there. Our mission continues. Together with those we serve, we continue to evolve and stay ahead of the needs of patients and health care systems.
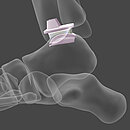
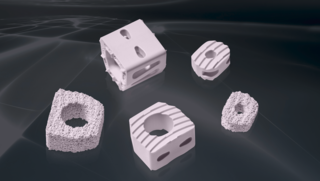
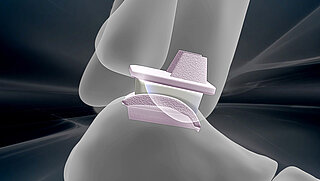
Today, our commitment to innovation is reflected in novel BIOLOX®delta products: two BIOLOX®delta ceramic-on-ceramic hip resurfacing systems and our new BIOLOX® CERAMIC Knee. Each has been granted FDA Breakthrough Device Designation.
Experience the future and explore what lies ahead.
BIOLOX®delta is a highly biocompatible and hypoallergenic ceramic material. It shows superior physico-chemical properties that result in excellent wettability and a very high wear resistance. BIOLOX®delta is safe in terms of metal ion release and pathogenic reactions to ceramic particles are highly unlikely. Citations for all statements can be found here.
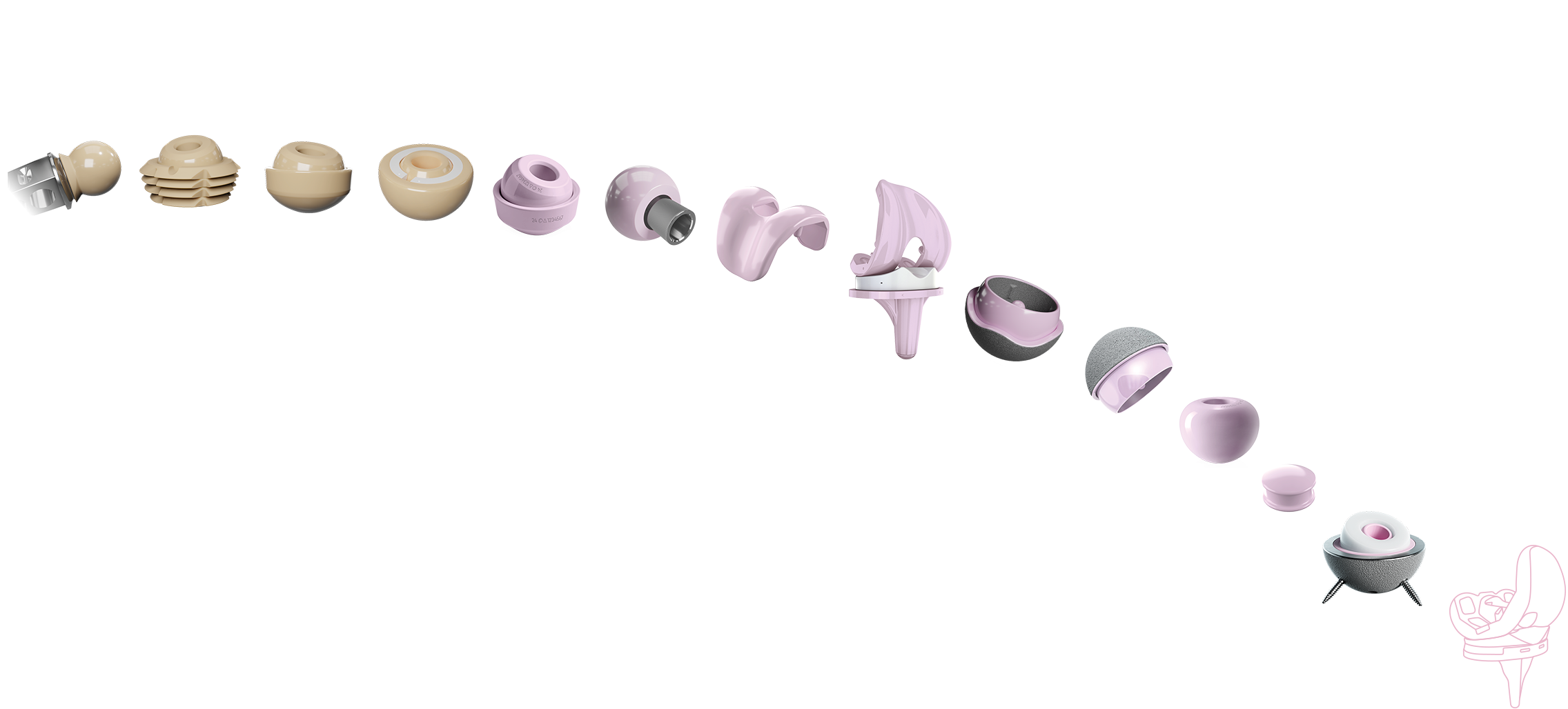 1BIOLOX®2Autophor Liners made of BIOLOX® ceramics3BIOLOX®forte ceramic-on-ceramic bearing4BIOLOX®DUO5BIOLOX®delta6BIOLOX®OPTION7BIOLOX®delta Ceramic Knee8BIOLOX®delta Ceramic Knee9BIOLOX®delta Ceramic-on-Ceramic Hip Resurfacing10BIOLOX®delta Ceramic-on-Ceramic Hip Resurfacing11BIOLOX CONTOURA®12BIOLOX®delta Core for Cervical Disc13BIOLOX®delta ceramic insert for Modular Dual Mobility System14BIOLOX® CERAMIC KNEE
1BIOLOX®2Autophor Liners made of BIOLOX® ceramics3BIOLOX®forte ceramic-on-ceramic bearing4BIOLOX®DUO5BIOLOX®delta6BIOLOX®OPTION7BIOLOX®delta Ceramic Knee8BIOLOX®delta Ceramic Knee9BIOLOX®delta Ceramic-on-Ceramic Hip Resurfacing10BIOLOX®delta Ceramic-on-Ceramic Hip Resurfacing11BIOLOX CONTOURA®12BIOLOX®delta Core for Cervical Disc13BIOLOX®delta ceramic insert for Modular Dual Mobility System14BIOLOX® CERAMIC KNEEAlumina skirted heads made of BIOLOX®
Alumina Ceramic: hipped, lasered, proofed
Bipolar Shell and Head
Zirconia, Platelet-Toughened Alumina Ceramic Composite
Sleeved Ceramic Head made of BIOLOX®delta
Multigen Plus Ceramic Knee, LIMA Corporate S.p.A.
BPK-S Ceramic Knee, Peter Brehm GmbH
H1® Ceramic Hip Resurfacing, Embody Orthopaedic Ltd.
ReCerf® Ceramic Hip Resurfacing, MatOrtho Limited
Anatomically Contoured Femoral Head
For cervical artificial discs
Breakthrough Device Designation granted to CeramTec by the FDA
Alumina skirted heads made of BIOLOX®
Alumina Ceramic: hipped, lasered, proofed
Bipolar Shell and Head
Zirconia, Platelet-Toughened Alumina Ceramic Composite
Sleeved Ceramic Head made of BIOLOX®delta
Multigen Plus Ceramic Knee, LIMA Corporate S.p.A.
BPK-S Ceramic Knee, Peter Brehm GmbH
H1® Ceramic Hip Resurfacing, Embody Orthopaedic Ltd.
ReCerf® Ceramic Hip Resurfacing, MatOrtho Limited
Anatomically Contoured Femoral Head
For cervical artificial discs
Breakthrough Device Designation granted to CeramTec by the FDA
For further information please contact our Head of Innovation, Dr. Henrich Mannel, via email.

For further information please contact our Head of Innovation, Dr. Henrich Mannel, via email.
For further information please contact our Head of Innovation, Dr. Henrich Mannel, via email.

For further information please contact our Head of Innovation, Dr. Henrich Mannel, via email.

For further information please contact our Head of Innovation, Dr. Henrich Mannel, via email.

For further information please contact our Head of Innovation, Dr. Henrich Mannel, via email.
Receive the latest news on bioceramics and current research findings directly via email.
Subscribe nowReferences
1. Sharplin P, Wyatt MC, Rothwell A, Frampton C, Hooper G. Which is the best bearing surface for primary total hip replacement? A New Zealand Joint Registry study. Hip Int. 2018;28(4):352-362. doi:10.5301/hipint.5000585.
2. Peters RM, Van Steenbergen LN, Stevens M, Rijk PC, Bulstra SK, Zijlstra WP. The effect of bearing type on the outcome of total hip arthroplasty. Acta Orthop. 2018;89(2):163-169. doi:10.1080/17453674.2017.1405669.
3. Maccauro G, Cittadini A, Magnani G, Sangiorgi S, Muratori F, Manicone PF, Rossi Iommetti P, Marotta D, Chierichini A, Raffaelli L, Sgambato A. In vivo characterization of Zirconia Toughened Alumina material: a comparative animal study. Int J Immunopathol Pharmacol. 2010;23(3):841-846. doi:10.1177/039463201002300319.
4. Cunningham BW, Hallab NJ, Hu N, McAfee PC. Epidural application of spinal instrumentation particulate wear debris: a comprehensive evaluation of neurotoxicity using an in vivo animal mode. J Neurosurg Spine. 2013;19:336-350. doi:10.3171/2013.5.SPINE13166.
5. Asif I M. Characterisation and Biological Impact of Wear Particles from Composite Ceramic Hip Replacements. [PhD thesis]. Leeds, UK: University of Leeds; 2018. etheses.whiterose.ac.uk/20563. Accessed March 6, 2020.
6. Beraudi A, Stea S, De Pasquale D, et al. Metal ion release: also a concern for ceramic-on-ceramic couplings? Hip Int. 2014;24(4):321-326. doi:10.5301/hipint.5000132.
7. Kretzer JP, Mueller U, Streit MR, et al. Ion release in ceramic bearings for total hip replacement: Results from an in vitro and an in vivo study. Int Orthop. 2018;42(1):65-70. doi:10.1007/s00264-017-3568-1.
8. Thomas P, Stea S. Metal Implant Allergy and Immuno-Allergological Compatibility Aspects of Ceramic Materials. Heidelberg, Germany: Springer-Verlag Berlin Heidelberg; 2015.
9. Tsaousi A, Jones E, Case CP. The in vitro genotoxicity of orthopaedic ceramic (Al2O3) and metal (CoCr alloy) particles. Mutat Res. 2010;697(1-2):1-9. doi:10.1016/j.mrgentox.2010.01.012.
10. Esposito C, Maclean F, Campbell P, Walter WL, Walter WK, Bonar SF. Periprosthetic tissues from third generation alumina-on-alumina total hip arthroplasties. J Arthroplasty. 2013;28(5):860-866. doi:10.1016/j.arth.2012.10.021.
11. Trieb K, Ullmann D, Metzinger K, et al. Prospective Comparison of a Metal-Free Ceramic Total Knee Arthroplasty with an Identical Metal System. Z Orthop Unfall. 2018;156(1):46-52. doi:10.1055/s-0043-118600.
We support you




 Since 1974
Since 1974