8 years, 316 cases, 0 HNJ failures: BIOLOX ® OPTION systems hold up in revision THA
Now, a new retrospective single-center study from the University of British Columbia, Canada by Dr. Don Garbuz (with contributors from the University of Warwick, UK) provides mean 7.7-year follow-up data on the use of this construct combining BIOLOX®𝘥𝘦𝘭𝘵𝘢 ceramic heads with titanium adapter sleeves in revision total hip arthroplasty (rTHA).
Ceramic heads are used for their favorable biological profile and ability to mitigate trunnion-related fretting/corrosion compared to CoCr heads.
Meanwhile, the titanium adapter sleeve allows placement of a ceramic head on a retained stem taper that may have minor damage. However, its introduction of additional metal-metal and metal-ceramic interfaces raises concerns about long-term integrity, corrosion risk, and femoral head-neck junction (HNJ) failure.
Previous clinical series and 𝘪𝘯 𝘷𝘪𝘵𝘳𝘰 work (including a 516-case series of the Mayo Clinic and other single-center cohort reports) have supported the short- to mid-term reliability of sleeved ceramic heads. Garbuz et al. offer additional evidence.
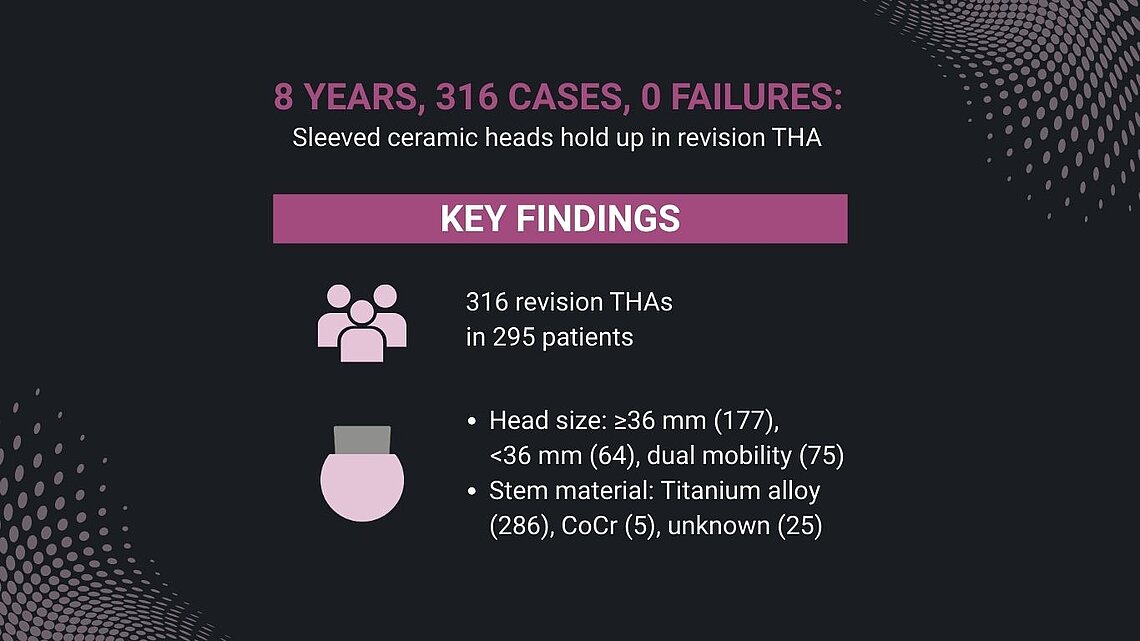
The study at a glance:
- 316 revision THAs in 295 patients (170 women, 125 men; mean age 65), 2011-2022
- Head size: ≥36 mm (177), <36 mm (64), dual mobility (75)
- Stem material: Titanium alloy (286), CoCr (5), unknown (25)
- Revision indications: ALTRs (45,6%), instability (28,5%), acetabular aseptic loosening (12%)
Results atmean 8-year follow-up:
- 100% failure-free survival of head-neck junction (HNJ)
- No re-revisions for HNJ failure observed
- 66 hips (22%) underwent re-revision at mean 1.8 years, mainly due to instability, infection, and aseptic loosening
- 81.1% all-cause re-revision-free survival at 5 years; 77.0% at 10 years
- Instability (39%), infection (26%), and aseptic loosening (12%) were the leading reasons for re-revision
Clinical takeaway
The authors conclude that new ceramic heads with titanium sleeves on retained femoral stems appear safe and reliable at mean 7.7-year follow-up, with no HNJ failures in this series. These results echo other mid-term series reporting excellent clinical outcomes with BIOLOX®OPTION systems with BIOLOX®𝘥𝘦𝘭𝘵𝘢 femoral heads. Most re-revisions are related to instability and infection instead of the sleeve construct.
Please check for regulatory approval in your country.
Disclaimer: Products are not registered/available in all countries.
References:
- Seah K, Howard L, Horwood N, Masri B, Garbuz D, Neufeld M. Is the Use of New Ceramic Heads with Titanium Sleeves on Retained Femoral Stems in Revision Total Hip Arthroplasty Associated with Femoral Head or Neck Junction Failure at Mean Eight-year Follow-up? J Arth. 24 November 2025. doi:10.1016/j.arth.2025.11.058
- Roberts, H.J., et al., New Ceramic Heads With Titanium Sleeves on Retained Femoral Components: Results of Over 500 Revision Total Hip Arthroplasties. J Arth 2024. 39(9S1): p. S183–S187.
- Simon, S., et al., Ceramic Heads With 12/14 Titanium Sleeves Used on Manufacturer-Non-Compatible Retained Femoral Components Do Not Lead to Implant Failure in Revision Hip Arthroplasty. J Arth 2025. 40(2): p. 475–479.
This text was created with the support of AI.