Key insights from the New Zealand Joint Replacement Registry Report 2025
Primary hip replacement:
Since its inception, 202,460 primary hip arthroplasties have been registered in the NZJR. From 2023 to 2024, total hip arthroplasties (THA) rose by 5%.
Fixation trends:
Uncemented fixation led the way in 2024 (now accounting for 51.7% of procedures, with 5,728 uses out of 11,078 total procedures), followed by hybrid (44.8%, with 4,965 uses) and cemented (<5%, with 332 uses).
Bearing surfaces:
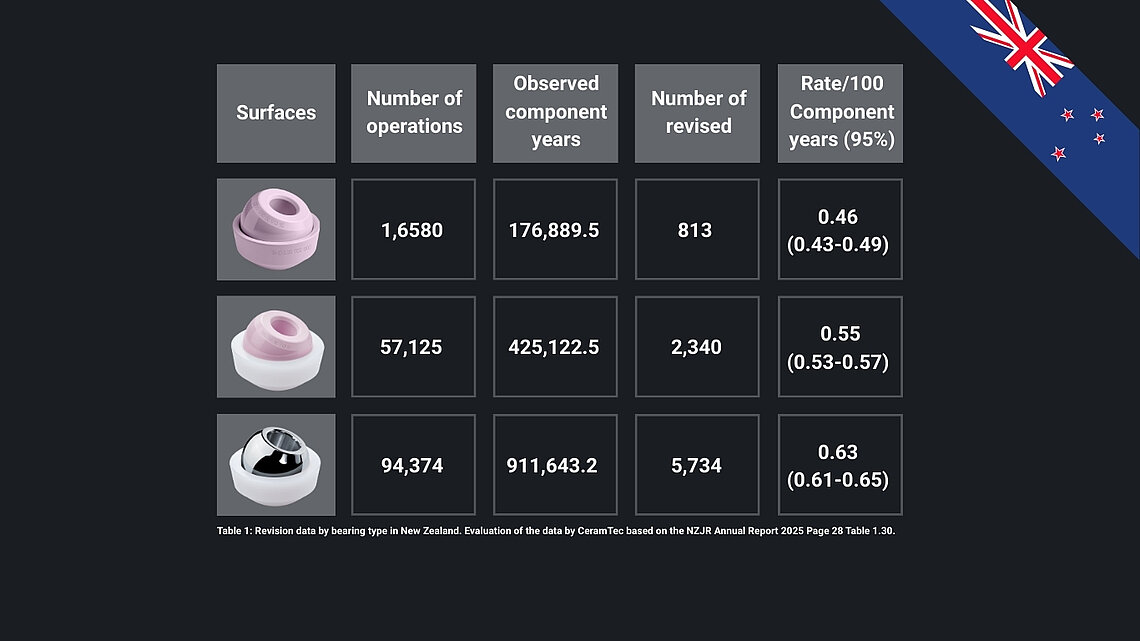
Ceramic-on-polyethylene (CoP) is now the predominant bearing surface (>60%), while metal-on-polyethylene (MoP) is ~20%. Ceramic-on-ceramic (CoC) is under 5%, showing the lowest revision rates in the registry data.
Head size preferences:
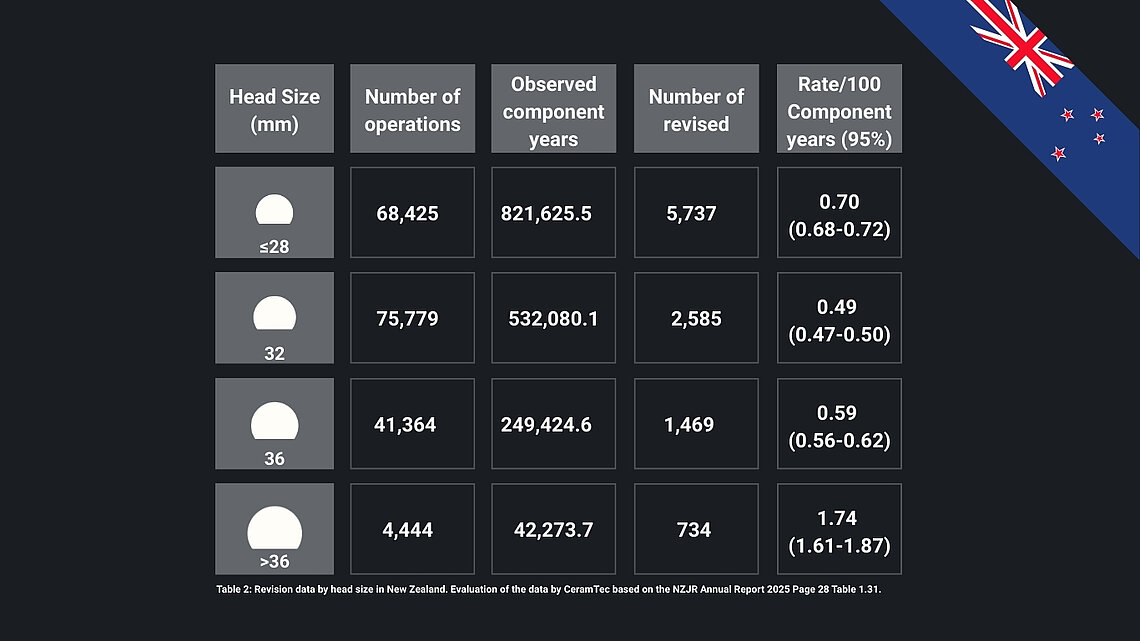
36mm heads are used in about half of cases, accounting for 90% of all procedures together with 32mm heads. ≤28mm and >36mm head sizes remain rare.
Revision:
In 2024, the top reasons for revision THA were deep infection (22.8%), dislocation (19.4%), femoral fracture (18.2%), femoral loosening (14.7%), acetabular loosening (12.5%), and unexplained pain (2.9%).
- Notably, revisions for deep infection have increased since 2012, while those for unexplained pain have dropped significantly.
- Between 1999 and 2024, 32,275 hip revision procedures were registered in the NZJR. 1,484 new hip revisions were recorded in 2024.
- CoC bearings show the lowest revision rates (0.46/100 component years), followed by CoP (0.55) and MoP (0.63). 32mm head sizes show the lowest revision rates among groups.
Patient-reported outcomes (PROMs):
The Oxford-12 questionnaire shows that at 6 months, 84% of patients report excellent or good function (mean score 40.3). High satisfaction levels are maintained at five-year intervals up to 20 years post-op.
For more details, refer to the full NZJR Annual Report 2025:https://www.nzoa.org.nz/sites/default/files/documents/DH9492_NZOA_AnnualReport2025_FINAL.pdf
Note: The NZJR Annual Report is an independent registry publication and reports aggregated outcomes across multiple implant manufacturers and bearing designs. The bearing-surface categories cited (e.g., CoC, CoP, MoP) are not limited to BIOLOX® components, and this post does not report outcomes for any specific brand or product.
Please check for regulatory approval in your country.
This text was created with the support of AI.
📖 Reference:
New Zealand Orthopaedic Association. Twenty-six-year report January 1999 to December 2024. The New Zealand Joint Registry. Accessed December 15, 2025.