A biocompatible material
- Is able to perform its desired function with respect to a medical therapy.
- Generates the most appropriate beneficial cellular or tissue response.
- Elicits no undesirable local or systemic effects in the recipient.
Read the FDA statement on their website.
Download the document “Biological Responses to Metal Implants” from the FDA website.
Appropriate cellular or tissue response
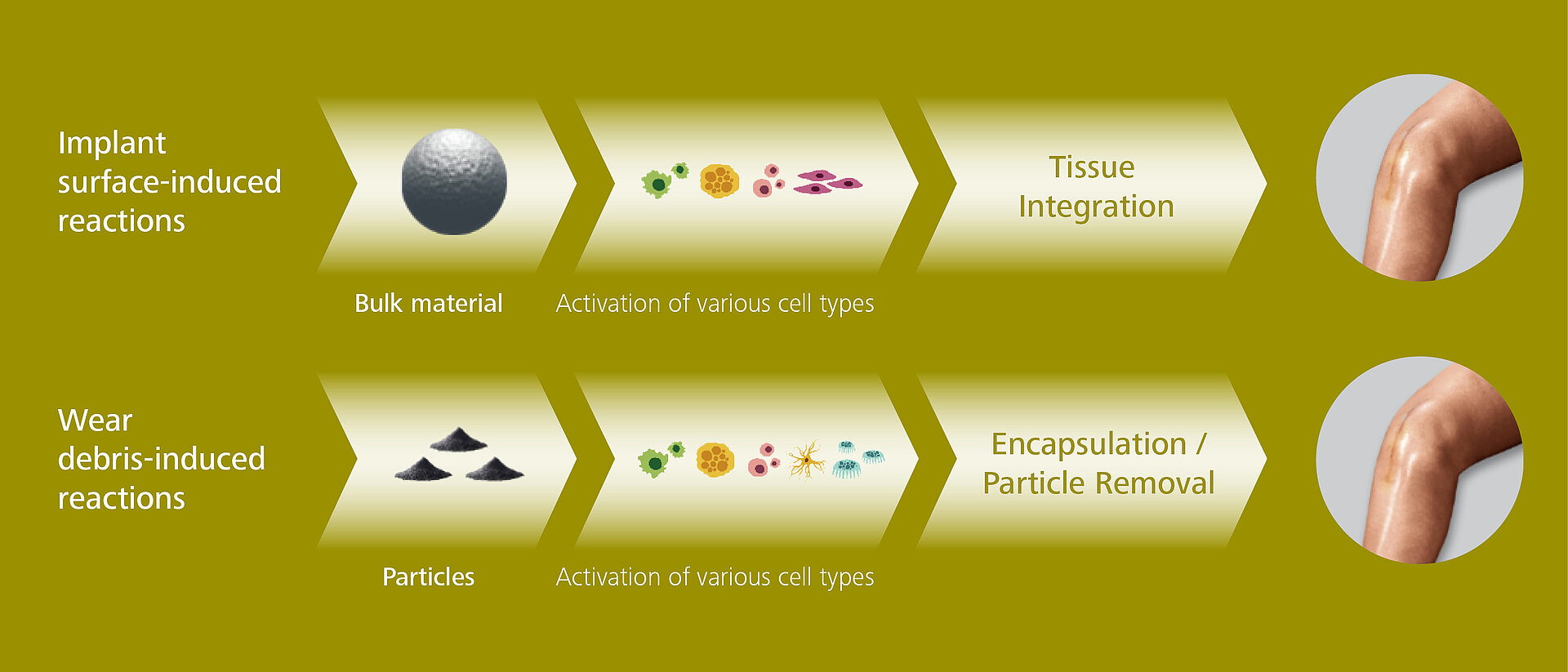
Appropriate and adequate immune response with a proper activation of the immune cells takes place, including a directed biological response, a good host-implant integration, a desired healing process as well as in the case of wear-in-duced immune reaction the possible encapsulation of wear particles and their removal.
Inappropriate cellular or tissue response
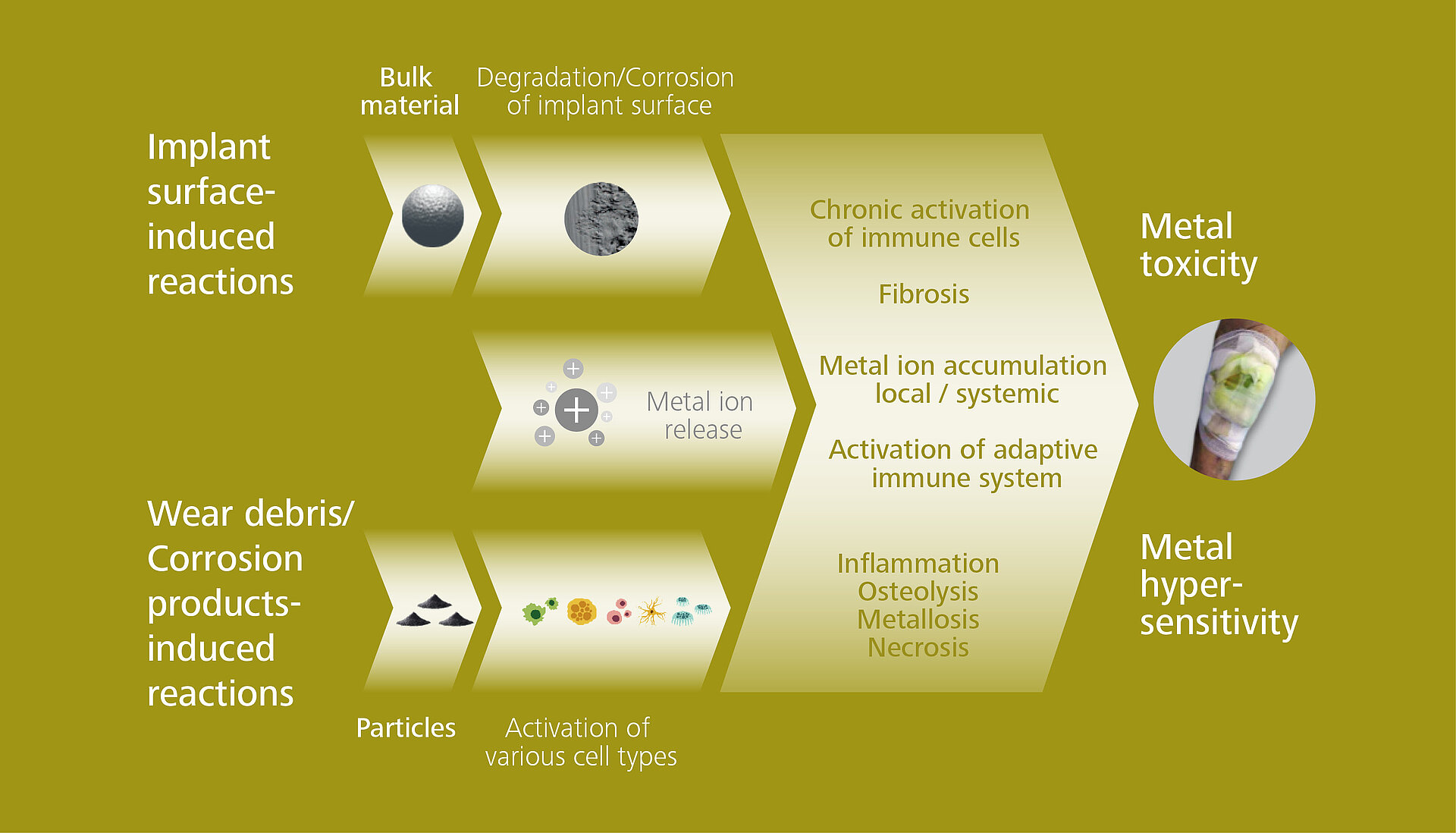
An implant material can activate and initiate an inappropriate immune response. For example, degradation and corrosion of the implant over time can trigger an elevated and uncontrolled immune response with a chronic activation of immune cells and fibrosis that can lead to implant failure.
Local and systemic toxicity
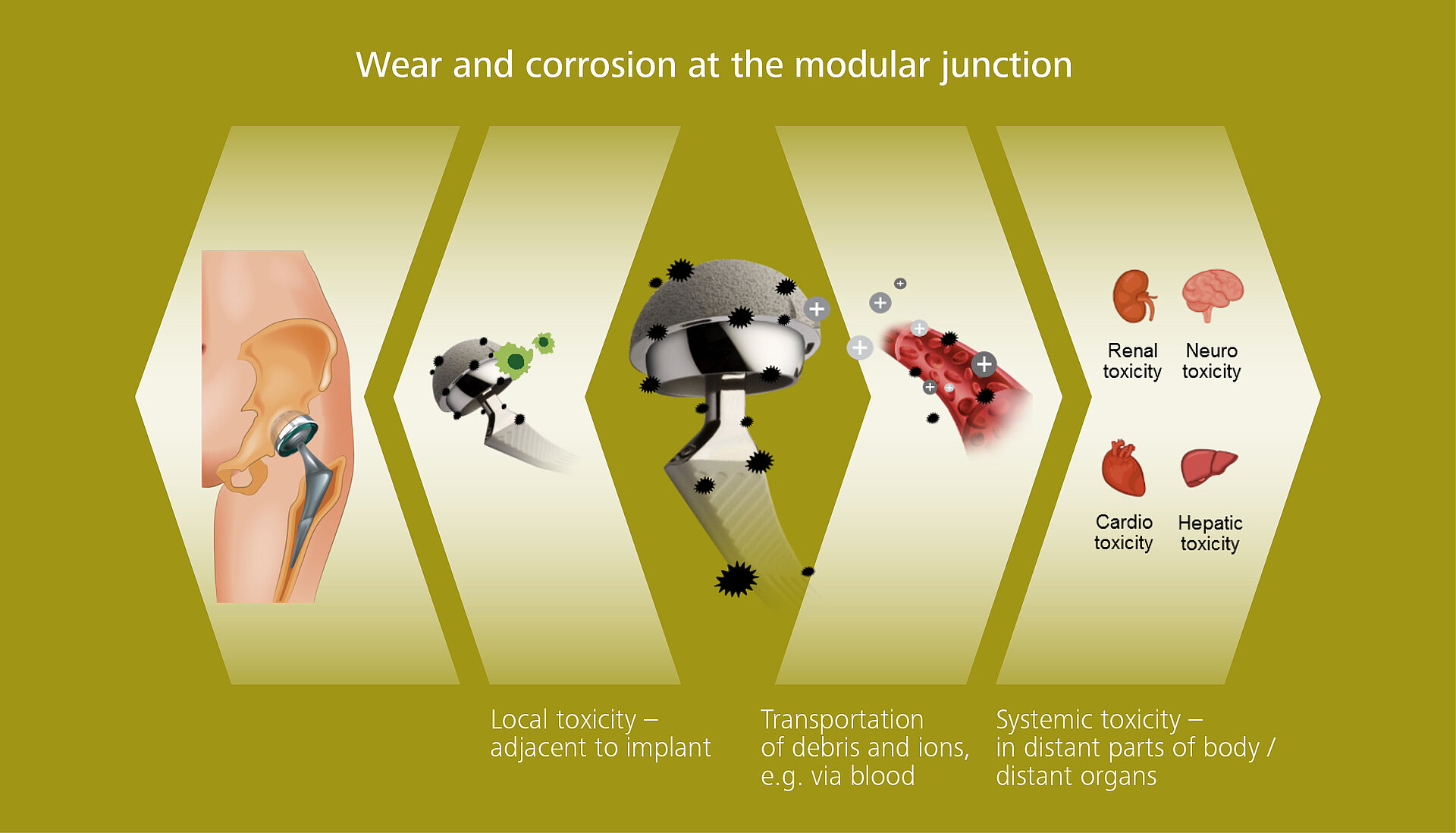
Metal ions that are eluted into the peri-implant space or generated by wear and corrosion at the modular junction can either accumulate locally within cells and tissues which surround the implant. However these metal ions can also spread systemically via their transportation through the blood stream and hence can accumulate in distant organs (e.g. liver, heart, spleen ...) that can lead to metal intoxication, which can cause long-term systemic complications to the organism.
CeramTec is committed to selecting and bringing to interested parties relevant articles on bioceramics related topics. The presented authors’ views and opinions are solely those of the authors of these publications. It is the focus and intent of CeraNews that CeramTec presents and comments on the authors’ views and opinions in a specific context. Such comments and editorials therefore solely express CeramTec’s views and opinions and not necessarily those of the quoted authors.
References
Ghasemi-Mobarakeh L. et al. Key terminology in biomaterials and biocompatibility. Current Opinion in Biomedical Engineering 2019, 10:45–50
Ong S.M. et al. (2015), MicroRNA-mediated immune modulation as a therapeutic strategy in host-implant integration, Adv. Drug Del. Rev., 88 p. 92–107
Man K. et al., (2017), Immunological response to total hip arthroplasty, J. Funct. Biomater., 8(3)
Dahms K et al. Cobalt intoxication diagnosed with the help of Dr House. Lancet. 2014 Feb 8;383(9916):574. doi: 10.1016/S0140-6736(14)60037-4
Swiatkowska I. et al. (2018) Synchrotron analysis of human organ tissue exposed to implant material. J Trace Elem Med Biol. 46: 128–137.