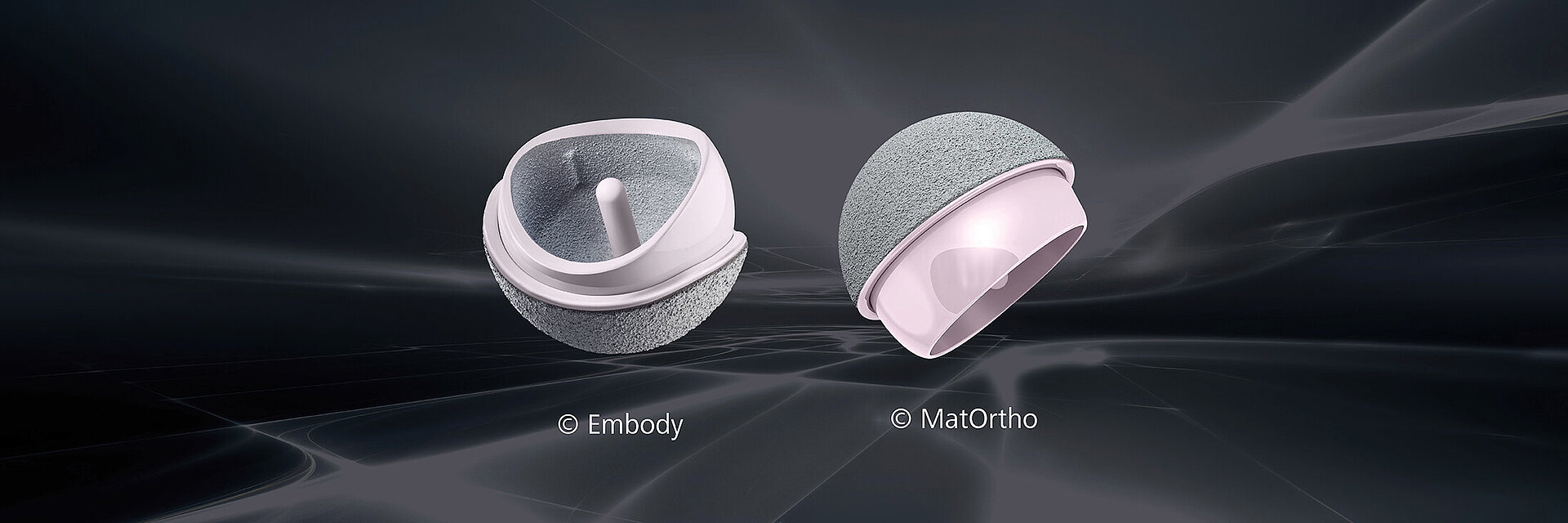
Making Resurfacing Biocompatible: The Ceramic Option
Removing metal from bearing surfaces eliminates the most important limitation of Hip Resurfacing Arthroplasty (HRA). Ceramic-on-ceramic bearing could be the (bio-) logical alternative: It does not release toxic metal ions and allows thin wall geometries necessary for HRA. Ceramic-on-ceramic HRA devices have the potential to offer the advantages of metal-on-metal resurfacing devices like the Birmingham and Adept Hip resurfacing system without Cobalt and Chrome ion release. Currently, two ceramic-on-ceramic hip resurfacing systems are undergoing clinical evaluations in a trial for approval.*
Ceramic: A benchmark for bearing couples behaviour
Ceramic materials have a number of natural advantages when used as implant components in the human body. They resist practically any chemical reaction in physiological conditions; they exhibit excellent biological behavior and they show low immunological response. Further, specialized high-performance ceramics are highly resistant to wear with minimal production of wear debris, high fracture strength and toughness, and a very low immune response – all prerequisites for long-term survival of ceramic implants1. Ceramic materials coated with vacuum-plasma-sprayed titanium for cementless osseointegration are also compatible with MRI procedures2.
Due to these technical attributes, surgeons around the world have opted for ceramic bearing components for millions of hip arthroplasty surgeries over the last four decades. Since its market introduction in the year 2003, the pink colored BIOLOX®delta ceramic has become the most widely used ceramic implant material in hip replacement defining a true benchmark in this field.
BIOLOX®delta is a zirconia-toughened alumina matrix composite ceramic with high fracture strength, excellent wear properties against both ultra-high molecular weight polyethylene and importantly against itself. Its introduction opened up new horizons for making complex geometries and a wider range of orthopedic innovations possible.
Fewer immune reactions, fewer bacterial infections
The biocompatibility of alumina matrix composites like BIOLOX®delta has been proven clinically, in in-vitro studies and in in-vivo animal studies6,7,8,9. BIOLOX®delta particles do not stimulate inflammatory responses, do not cause DNA damage, nor oxidative stress in human cells in clinically relevant doses i.e. the material and its debris are neither cytotoxic nor genotoxic6. Furthermore, recent in-vitro studies and analyses of explants have demonstrated that bacterial adhesion on BIOLOX® ceramic surfaces is reduced in comparison to other bearing materials. Studies also show a reduced biofilm formation on BIOLOX® surfaces compared to metal and polymer surfaces10,11.
Ceramic-on-ceramic HRA Systems
Due to very high fracture strength, toughness and wear resistance, BIOLOX®delta appears well-suited for use in hip resurfacing. Thin-walled ReCerf® acetabular cups made of this material have shown very high resistance to critical impact loads in a laboratory study under extreme conditions. All components passed a lateral fall simulated impact test without fracture and showed no visible damage. Eighty four percent of cups further survived a high velocity car crash simulated impact known to be sufficient to fracture the pelvis; beyond this loading some fractures of the cup edges could be produced4.
Both systems comprise monoblock femoral and acetabular components. These consist of BIOLOX®delta ceramics with vacuum-plasma-sprayed titanium and hydroxyapatite for cementless fixation. This type of textured surface is highly stable and biocompatible at the same time. It has been successfully used for decades on bone-facing surfaces of orthopaedic implants, enabling good primary fixation as well as long-term stability via osseointegration.2 MatOrtho utilizes cement fixation for the ReCerf® HRA head to match the fixation method used for the majority of HRAs to date.
The H1® (Embody Ltd.) and the ReCerf® (MatOrtho Ltd.) ceramic hip resufacing devices are both undergoing Clinical Investigations approved by the UK Health Research Authority. H1® was implanted for the first time in 2017, ReCerf® in September 2018. The H1® has passed the phase safety study and patient enrollment has been concluded. The ReCerf® trial is underway with a number of implantations designated for special access by specialist surgeons under their local approval systems with all cases followed assiduously. To date 412 ReCerf® HRAs have been implanted.
The H1® is investigational device tested in a clinical study approved by MHRA and NHS, respectively. The ReCerf®, manufactured by MatOrtho Limited, is currently under an approved clinical MHRA investigation.
Resurfacing for smaller anatomies and women?
The reasons for data showing women to have lower implant survival with metal-on-metal HRA remain obscure. A number of reasons have been postulated and some high-volume surgeons have shown better outcome survival in women than men. There is little doubt that the reasons are multifactorial, and it should be borne in mind that HRA was introduced to a large population concurrently with surgeon learning and wide indications for HRA being factors. Most HRA devices have fallen by the wayside during the demographic period with some performing very poorly more so than others and the BHR was for many of the early years forming a high volume of data. For many years this implant was only available in 4 mm size increments dictating a method of head size choice which consequently induced what is now recognized as undersizing in many cases. This will have led to greater difficulty for ideal placement and a strong possibility of neck impingement which is in turn associated with edge loading cup placement and increased metal ion release.
Thus, todays HRA geometry, sizing operative indications, and technique training is very different for which statistics will emerge in due course but needs to have a restart from about 10 years ago. There is little doubt however that removing metal ions will be of benefit for the technology. The articulating surfaces feature all the proven qualities of the BIOLOX®delta ceramic material. The most important are the lowest wear rate of all bearing materials, no metal ions, minimal wear debris generation, no polyethylene particles, superior lubrication and extremely low friction as well as precisely defined clearances. Ceramic materials are highly resistant to corrosion. As a result, issues that can lead to elevated wear rates and a high risk of adverse reaction to metal debris in metal-on-metal implants are ruled out by using a ceramic-on-ceramic bearing.
A change from metal to BIOLOX®delta was shown to maintain low wear under tests known to accelerate the wear rate of metal-on-metal. Wear tests simulating microseparation conditions in large diameter ceramic resurfacing bearings have reported low wear rates; 47 times lower than that reported for metal-on-metal bearings under these same adverse conditions: ‘The low wear rate and highly biocompatible nature of this ceramic potentially offers an advantage over current meta-on-metal hip resurfacing designs and may represent the next generation of resurfacing devices’5. While there is currently limited data on these two implant devices it is possible that the indications may be widened clinically for what has been a restricted group more recently with metal-on-metal resurfacing devices including inclusion for smaller patients and women. With the use of ceramic- on-ceramic bearings, HRA could once again become a realistic option for patients with good bone stock in hip arthroplasty.
References
1. Piconi C, Porporati A A. Bioinert Ceramics: Zirconia and Alumina. Handbook of Bioceramics and Biocomposites.doi:10.1007/978-3-319-09230-0_4-1. Springer International, 2015.
2. Hanawa T (2019) Titanium–Tissue Interface Reaction and Its Control With Surface Treatment. Front. Bioeng. Biotechnol. 7:170. doi:10.3389/fbioe.2019.00170.
3. Porporati A A, Piconi C, Mettang M, Deisinger U, Reinhardt C, Pitto R. Ceramics for artificial joints: The relevance of material biocompatibility. in: Bioceramics.doi.org/10.1016/B978-0-08-102999-2.00012- 0. Elsevier, 2021.
4. de Villiers D, Collins S. Resistance of a novel ceramic acetabular cup to critical impact loads. J Engineering in Medicine 2020. doi:10.1177/0954411920941383.
5. de Villiers D, Collins S. Wear of large diameter ceramic-on-ceramic hip bearings under standard and microseparation conditions. Biotribology. 2020; 21:100117. doi:10.1016/j.biotri.2020.100117.
6. Asif I M. Characterisation and Biological Impact of Wear Particles from Composite Ceramic Hip Replacements. [PhD thesis]. Leeds, UK: University of Leeds; 2018. etheses.whiterose.ac.uk/20563. Accessed March 6, 2020.
7. Cunningham BW, Hallab NJ, Hu N, McAfee PC. Epidural application of spinal instrumentation particulate wear debris: a comprehensive evaluation of neurotoxicity using an in vivo animal model. J Neurosurg Spine. 2013;19(3):336-350. doi:10.3171/2013.5.SPINE13166. Epub 2013 Jun 28.
8. Kretzer JP, Mueller U, Streit MR, et al. Ion release in ceramic bearings for total hip replacement: Results from an in vitro and an in vivo study. IntOrthop. 2018;42(1):65-70. doi:10.1007/s00264-017-3568-1.
9. Porporati AA, Piconi C, Mettang M, Deisinger U, Reinhardt C, Pitto R. 12 - Ceramics for artificial joints: The relevance of material biocompatibility. In: Osaka A, Narayan R, eds. Bioceramics From Macro to Nanoscale. Elsevier Series on Advanced Ceramic Materials. Elsevier; 2021. doi:10.1016/B978-0-08-102999-2.00012-0. Accessed November 10, 2020.
10. Pitto RP, Sedel L. Periprosthetic joint infection in hip arthroplasty: Is there an association between infection and bearing surface type? Clin Orthop Relat Res. 2016;474(10):2213-2218. doi:10.1007/s11999- 016-4916-y.
11. Lenguerrand E, Whitehouse MR, Beswick AD, et al. Risk factors associated with revision for prosthetic joint infection after hip replacement: a prospective observational cohort study. Lancet Infect Dis. 2018;18(9):1004-1014. doi:10.1016/S1473-3099(18)30755-2.